Abstract
Background
Chemotherapy-based approaches remain an essential component of current adult acute lymphoblastic leukemia (ALL) treatment. Poor outcomes in older patients with ALL result from the disease's adverse biology and poor tolerance to chemotherapy. Pediatric-inspired protocols have been used over the past 20 years in our center for patients > 60-year-old, both Philadelphia negative (Ph-), and Philadelphia positive (Ph+). Historically, Ph+ patients have been believed to have inferior outcomes. Our goal was to estimate the outcomes of this subgroup of patients and to compare outcomes between Ph- and Ph+ patients.
Methods
All newly diagnosed patients with ALL aged > 60 years, and receiving induction chemotherapy with a modified pediatric-inspired regimen between January 2002 and January 2022 were included. The treatment regimen consisted of induction, central nervous system prophylaxis, seven 3-week cycles of intensification, and 24 3-week cycles of maintenance (PM DFCI-based protocols). Only Ph- patients received asparginase. All Ph+ patients received a Tyrosine Kinase Inhibitor (TKI) during all phases of treatment and continued indefinitely after maintenance. There were adjustments in the type of drugs and dosages over the years. Standard descriptive statistics were used. Ninety-five percent confidence intervals were provided for estimates of interest where possible. Fisher's exact test was used to assess the impact of categorical covariates. P < 0.05 were considered to indicate a significantly different result. Kaplan-Meier survival estimate was used to evaluate Leukemia free survival (LFS) and overall survival (OS).
Results
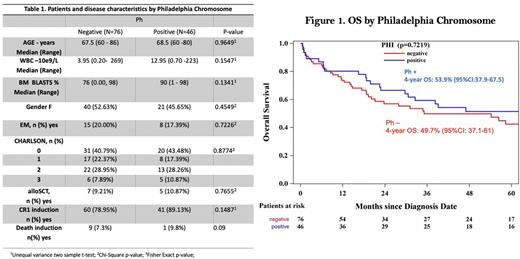
A total of 122 patients were included: Ph- B-ALL, 50% (N=61); Ph+ B-ALL, 38% (N=46); Mixed Phenotype Acute Leukemia (MPAL), 4% (N=5); and T-ALL, 8% (N=10). Out of the Ph+ patients, only one had an MPAL diagnosis, the rest of them had B-ALL diagnoses. Patient and disease characteristics by Ph status are presented in table 1.
For the whole cohort, the complete remission rate after induction (CR) was 83% (N=101), and allogeneic stem cell transplantation (alloSCT) in CR1 was carried out for 10% (N=12). Ten patients (8.2%) died during induction and the cumulative incidence of relapse (CIR) at 1-year was 11.7 % (95% CI 6.7 - 18.2). CR, mortality at induction, alloSCT, and CIR rates were similar in the Ph- and Ph+ groups.
With a median follow-up of 52 months (range 4-209 months) among survivors, the median OS was 54 months (95%CI: 28-82). The 1-year and 4-year OS rates were 76% (95%CI: 68-83%) and 51.2% (95%CI: 41.4-60.2%), respectively. Leukemia-free survival (LFS) at years 4 years was 41.9% (95%CI: 32.7-50.9).
There were no statistical differences in median OS and LFS between Ph- and Ph+ patients. OS at 4 years for patients with Ph- ALL was 49.7% (95%CI: 37.1-61) vs 53.9% (95%CI:37.9-67.5) in Ph+ ALL patients (p=0.72) (Figure 1). At 4 years LFS was 40.8% (95%CI: 29 - 52) for Ph- patients and, 43.6% (95%CI: 28 - 57) for the Ph+ population (p=0.68).
Conclusion
Pediatric-inspired chemotherapy protocols with appropriate dose adjustments for age are associated with good outcomes in older ALL patients. Clinical and disease characteristics and outcomes were similar between Ph- and Ph+ ALL patients. We aim to further define the optimal therapy for this group of patients with this challenging disease.
Disclosures
Perusini:Pfizer: Consultancy. Gupta:AbbVie: Consultancy, Other: Participation on a Data Safety or Advisory board; BMS Celgene: Consultancy, Honoraria, Other: Participation on a Data Safety or Advisory board; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Honoraria; Roche: Other: Participation on a Data Safety or Advisory board; Sierra Oncology: Consultancy; Pfizer: Consultancy, Other: Participation on a Data Safety or Advisory board; Novartis: Consultancy, Honoraria. Richard-Carpentier:Taiho: Other: Advisory Board Participation; BMS: Other: Advisory Board Participation; Astellas: Consultancy, Honoraria, Other: Advisory Board Participation; AbbVie: Consultancy, Honoraria, Other: Advisory Board Participation; Pfizer: Consultancy, Other: Advisory Board Participation. Yee:Takeda: Consultancy; Janssen: Research Funding; Abbvie: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Shattuck Labs: Consultancy; TaiHo: Consultancy; Astex: Research Funding; Pfizer: Consultancy; Jazz: Consultancy, Research Funding; GlaxoSmithKline: Consultancy; Karyopharm: Research Funding; F. Hoffmann La Roche: Consultancy, Research Funding; Bristol-Myers Squibb/Celgene: Consultancy; Gilead: Research Funding; Treadwell: Research Funding; Geron: Research Funding; Forma Therapeutics: Research Funding; Astellas: Consultancy. Schuh:Servier: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Teva Pharmaceutical Industries: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Phebra: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlycoMimetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal